Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Antioxidant and Anticancer Effects of Thymoquinone on Colorectal Cancer Cells
*Corresponding author: Trabelsi Mounir, Cytogenetics Laboratory, Molecular Genetics and Biology of Human Reproduction, Tunisia.
Received: June 24, 2022; Published: July 26, 2022
DOI: 10.34297/AJBSR.2022.16.002283
Abstract
Colorectal Cancer (CRC) is one of the most leading causes of death in the world and one of the major public health problems. Despite the great advances in cancer therapy, the incidence and mortality rates of CRC remain high. Therefore, there’s a need for more efficient and less toxic cancer treatment strategies. Herbs and spices could provide potential anticancer candidates. Herein we investigate antioxidant and anticancer activities of thymoquinone (TQ). Anticancer activity against HCT116 and HT29 was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lactate dehydrogenase (LDH) assays while antioxidant activity was assessed by DPPH and ABTS tests. For apoptosis study, Hoechst test were used. TQ induce HCT116 and HT29 cell cytotoxicity and apoptosis without affecting HCEC, non-tumoral cells. This study is the main step to develop a highly active and multicomponent anticancer drug from natural sources.
Keywords: HCT116; Thymoquinone; Apoptose; Antioxidant; Anti-proliferative; Colorectal cancer
Abbreviations: CRC: Colorectal Cancer; TQ: Thymoquinone; DDPH: 1,1-diphenyl-2-Picrylhydrazyl Radical; LDH: Cytosolic Enzyme Lactate Dehydrogenase; DMEM: Dulbecco’s Modified Eagle Medium; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Introduction
In terms of incidence and mortality, colorectal cancer ranks 3rd among cancers after prostate and lung cancer for men and the third most common cancer after breast and lung cancer for women in the United States [1]. Colorectal carcinogenesis is characterized by uncontrolled cell growth in the colon and rectum. It thus designates the transition from the healthy situation of a tissue to adenomas and adenocarcinomas. This complex and multi-genetic transition can take several decades. At the level of the colon, most cancers are said to be sporadic (without family history) and only 5 to 10% are of a genetic form. Medicine has tried to find anti-cancer therapies like chemotherapy and radiotherapy [2]. However, their toxicities towards rapidly renewing normal cells, such as bone marrow cells, and their lack of specificity cause many adverse effects that can be fatal [3]. Several studies have shown that the toxicity and failure of cancer chemotherapy are expressed by the generation of free radicals and the peroxidation of membrane lipids, which causes very severe hematological toxicity, hence the need to seek new drugs with low side effects [3,4]. In recent years, many scientists have advocated a return to traditional medicine [5]. This popular medicine, practiced by man since antiquity, is based on the use of so-called medicinal plants, vegetables, and fruits as sources of natural substances active in the treatment of most diseases [6]. According to the WHO, more than 80% of the world’s population uses medicinal plants to treat various health problems [7].
Several studies have shown that medicinal plants are very rich in molecules with biological activity, among them we can cite many different herbs and spices depending on the cultures and societies that use them [8]. Some of these foods are rich in nutrients and ingredients whose hidden pharmacological qualities range from anti-inflammatory to anti-cancer [9]. And as the father of medicine Hippocrates said “Let food be your medicine and let your medicine be in your food” [10]. This famous phrase of Hippocrates, centuries before Jesus Christ, is still relevant and remains valid for us today. Thyme or Nigella sativa is a plant of the Lamiaceae family originating from the Mediterranean basin. The Egyptians used it to embalm their dead while the Romans used it to perfume their living rooms. It is also used culunar in many dishes in the Middle East [11]. Several studies have demonstrated its anticancer, antifungal, and antibacterial power [7]. According to the work of Kubatka et al. [12], this plant allows the overexpression of certain proapoptotic genes such as Bax and the under expression of CD44, in the case of breast cancer, while in the case of colon cancer there is an inhibition of proliferation, invasion and cell migration in a dose-dependent manner with increased synthesis of caspases 3 and 7 [13]. The objective of the present study is to evaluate the antioxidant, proapoptotic and anticancer activities of TQ, active molecule of Nigella sativa, a spice used in Mediterranean cuisine, using colon cancer cell cultures (HCT116 and HT29)). This research is undertaken to examine, in an indirect way, the association between dietary habits and the rate of increase in colorectal cancer.
Materials and Methods
Materials
TQ were purchased from Sigma Chemicals Co. (USA), stored at 2-4°C away from sunlight. To treat cells, these compounds were dissolved directly in the cell culture medium (DMEM).
DPPH and ABTS Radical Scavenging Assay:
The antioxidant activity of compounds wasevaluated using DDPH and ABTS according to the method reported by Kartal and co-workers [14]. DDPH method is based on the ability of 1,1-diphenyl-2-picrylhydrazyl (DPPH) to decolorize in the presence of antioxidants. Briefly, 180μL of the test compound at concentrations ranging from 0.009μg/ml to 0.312 μg/ml were mixed with 0.1mL of the DPPH solution (0.2mg/mL in ethanol). The absorbance was determined at 515nm after 2, 5, 10, 15, 20 and 30 min of incubation at room temperature. Ascorbic acid (Vit. C) was used as a positive control. The percentage of inhibition of DPPH oxidation was calculated according to the following formula. ABTS radicals were freshly prepared by reacting 7.4mM ABTS with 2.6mM potassium persulfate (Sigma-Aldrich) for 24h in the dark at room temperature. Then, 1 mL of ABTS radical solution was mixed with 1mL of compounds at concentrations ranging from 0.009μg/ml to 0.312μg/ml and allowed to stand for 20min at room temperature. The ABTS radical solution mixed with ethanol in the absence of compounds was used as the control. The absorbance was measured at 750nm. The antiradical activity was expressed as IC50: the dose of TQ required inducing 50% inhibition. The lower IC50 value corresponds to a higher antioxidant activity of compound. The ability to scavenge the radicals was calculated using the following equation: % inhibition= [(A0-A1)]/A0 x 100, Where A0 was controlled absorption and A1 was test compound absorption.
Culture and treatments of cells: Human colorectal carcinoma cell lines HCT116 and HT29 were purchased from LGC standards S.r.l (Italy) and were cultured in DMEM containing 10% (v/v) heat inactivated fetal bovine serum, and 1% antibiotic (penicillin/ streptomycin). Human corneal epithelial cell line (HCECB4G12) was purchased from Biologically Compatible Substances Research Laboratory Monastir, Tunisia and were grown in DMEM supplemented with fetal bovine serum (3%), 1% insulin transfer in-selenium and 40 μg/mL gentamicin. The incubation of all cells was performed at 37°C in a humidified atmosphere containing 5% CO2.
Antiproliferative Activity Assay: The cytotoxicity of TQ in HCT116 cells, HT29 cells and HCEC B4G12 cells were analyzed by colorimetric 3-(4,5-dimethyl-2-thiazolyl-)-2,5-diphenyl- 2H tetrazolium bromide (MTT assay). MTT salt is reduced to formazan by the mitochondrial enzyme succinate dehydrogenase in the metabolically active cells and was performed on all cells as previously described [1]. Briefly, cells were plated in 96-well culture plates at a density of 1×104cells/ well and treated for 24h with different concentrations of TQ (12.5; 25 and 50 μg/ml for each compound). Cells used as negative control were treated only with cell medium (DMEM). After the addition of MTT (0.5mg/ml), cells were incubated at 37°C for 4h. After 4h incubation, the culture medium was discarded by gentle aspiration and replaced by 200μL of DMSO to dissolve the formazan crystals. The amount of formazan product was measured at 570nm and 650nm using a microplate reader. The number of viable cells corresponds to the production of formazan. All the reactions were performed in triplicate. Measured data of cellular viability were normalized using viability values of untreated control cells (100%).
Cell viability was calculated and expressed as percentages according to the following equation: Cell viability (%) = [Sample / Control] x 100
LDH-Cytotoxicity Assay:Cells were seeded in 96-well culture plates (5x104cells/well). After being incubated for 24h, cells were treated with different concentrations of samples for 48h. Lactate dehydrogenase (LDH) activity was determined using the LDHCytotoxicity Colorimetric Assay Kit from Bio Vision (Milpitas, CA, USA) following the manufacturer’s protocol. All the reactions were performed in triplicate. The absorbance of LDH in the culture medium was measured at 340nm using a microplate
Nuclear Morphology Detection using Hoechst 33342:HCT116 and HT29 cells (5×104cells) were plated in 60mm2 culture dishes and incubated in 5% CO2 incubator at 37°C for 24h. After incubation, the cells were treated with different concentrations of samples while negative control was treated with vehicle DMSO for 48h. The cells were then harvested and washed with PBS and subsequently stained with Hoechst 33342 (40μg/mL) at room temperature in the dark for 30min. Then, the cells were observed under inverted fluorescence microscope. Three hundred cells were examined for each sample.
Statistical analysis:SPSS Version 20 was used to perform the statistical analysis. Data are presented as the mean±standard error (SE) of triplicate experiments unless otherwise stated. Testing of parameters was carried out using Student’s t-test and one-way variance analysis. p<0.05 was considered statistically significant.
Results
The Antioxidant Activities
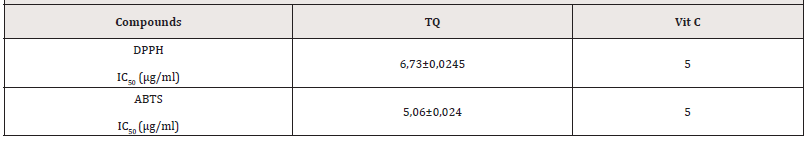
Antioxidants are of paramount importance in cellular functioning and play a crucial role in the prevention of cellular cancerization. To determine the antioxidant activities of thyme, we used 2 tests. These tests measured the activity of scavenging stable radicals [1]. The results of the DPPH scavenging activity of TQ are shown in Table 1. 6,73μg/ml of TQ is needed to obtain a IC50 for the DDPH and 5,06μg/ml with the ABTS test. This antioxidant activity is very close, but remains lower than that of Vitamin C, renowned for its great antioxidant activity. However, the DPPH radical scavenging activities were slightly lower than the ABTS radical scavenging activities. This difference could be due to their different mechanism of action and their different media. In fact, differences between DPPH and ABTS radical scavenging activities can be ascribed to reaction media. DPPH assay is conventionally conducted under 50% ethanol/water, whilst ABTS assay is carried out in aqueous conditions. Besides, flavonoids solubility in both media should be taken into consideration [15,16]. Several studies have also comprised the apoptotique role of TQ on CRC cells [17,18] but, to our knowledge none of them show the antioxidant effect (Table 1).
Effect of TQ on the Growth of Human Cancer Cells
After the proven antioxidant effect of TQ, we wanted to test its effect on cell proliferation to assess a possible cytotoxic effect. We used the MTT test on the 2 cell lines HCT116 and HT29. TQ causes a significant decrease in the viability of the 2 cell lines (Figure 1). This decrease is dose dependent. At a dose of 50μg/ml, TQ leads to the death of more than 40% of HCT116 cells and 35% of HT29 cells. Is, this cytotoxic effect specifically directed against cancer cells or is it toxic towards all cells, even healthy ones. To verify this “anti-cancer” effect, we measured the cytotoxic activity of TQ on non-malignant colonic epithelial cells HCEC-B4G12 (Figure 1). No decrease in viability was observed, suggesting a specific action on cancer cells. The anti-proliferative effect of cancerous or healthy cells, evaluated by the MTT clearly shows that TQ has a real promising cytotoxic effect on cancerous cells but remains without effect on healthy HCEC cells. A significant difference was observed particularly at the doses of 25 and 50μg-ml. These results are consistent with the previous findings reporting the antiproliferative and apoptosis induction effects of TQ against different cancer cell lines including adenocarcinoma, CRC cells, and breast cancer cells [16].
LDH-Cytotoxicity Assay
Injured or damaged cells release, among other things, LDH, an enzyme important in the metabolism of sugars and their conversion into energy. Considered as a biomarker of cytolysis, LDH is measured by a colorimetric test. The absorbance was analyzed at a wavelength of 340nm. The data is expressed as an average measured over 3 tests. Its presence, important, is proportional to the degree of cellular cytolysis. The cell culture medium was collected after 48h of treatment with the different chosen concentrations (Figure 2). As shown in Figure 2, an increase in the presence of LDH in the culture medium of the cells treated with TQ is noted at all the doses used and this effect is dose dependent. As indicate, at a dose of 50μg/ml the LDH released by the HCT116 cells is 2 times higher than that measured in the controls who did not receive any treatment. These results confirm the cytotoxic effect of TQ, and this effect is more marked on the cells of the adenocarcinoma.
Nuclear Morphology Detection using Hoechst 33342
The various tests carried out so far have indicated that TQ has an antioxidant effect and causes preferential cytotoxicity of adenocarcinoma cells. Cell death occurs in 2 ways, apoptosis or necrosis source of inflammation. For a product to claim an anticancer effect, the cytotoxicity generated must be of the apoptosis type. In our case, are we in the presence of an apoptosistype cytotoxicity? To answer this question, we used Hoechst staining which preferentially marks the compacted DNA of cells in apoptosis. Figure 3 shows that TQ at a dose of 50μg/ml leads to the death, by apoptosis, of more than 40% of the HCT 116 cells (Figure 3). At the same doses, the apoptotic effect of TQ on HT29 cells is always more discreet. As, we have just seen, the TQ shows a rather interesting apoptotic effect on the 2 cell lines used with a better effect on the HCT116 cells.
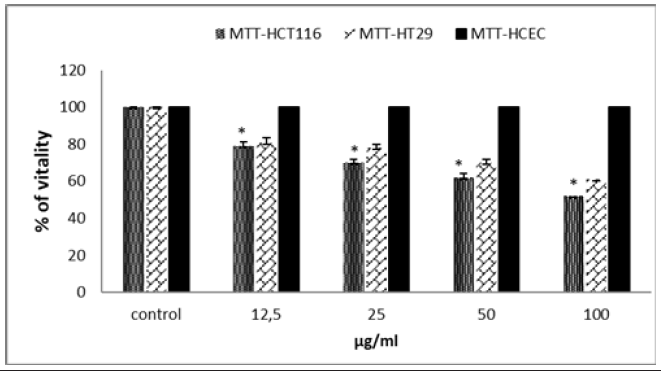
Figure 1: Effect of TQ on HCT116 cells, HT29 cells proliferation and on non-tumoral cells HCEC. Cell proliferation inhibitory activity was evaluated by the MTT assay. Data are expressed as means±standard error of the mean. Note: Indicates a significant difference (p<0.05).

Figure 2: Apoptotic cell death of HCT116 cells upon treatment with TQ for 48h. Effect of TQ on the release of LDH in HCT116 cells. The cell culture medium was collected after 48h of treatment with the indicated concentrations of all tested compounds. The cytotoxic was assessed biochemically by an increase in LDH content in the supernatant in a dose-dependent manner. Light absorptions were analyzed at 340nm. Data are expressed as means±standard error of mean, n=3. Note: Indicates a significant difference (p<0.05).
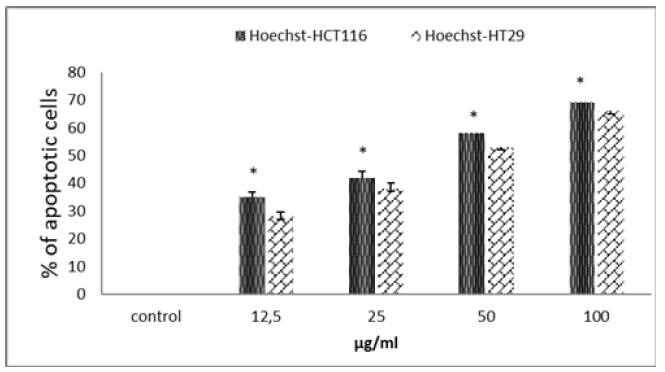
Figure 3: Apoptotic cell death of HCT116 and HT29 cells after DNA Hoechst 33258 staining upon treatment with TQ for 48h. The cell culture medium was collected after 48h of treatment with the indicated concentrations of all tested compound. Data are expressed as means±standard error of mean, n=3. Note: Indicates a significant difference (p<0.05).
Discussion
Numerous studies have shown that there is a correlation between regular consumption of fruits and vegetables and the prevention of lifestyle disorders such as cancer [19]. It is generally believed that diet and nutrient factors act as pro and antitumor risk modifiers in the whole process of several stages of colorectal tumorigenesis [20]. Nigella sativa is a plant widely used in Mediterranean cuisine. This plant appears to be an antiproliferative agent and appears to possess many desirable qualities for preventing cancer and may have great potential in the prevention of colorectal cancer [21]. Through this work we wanted to test the effect of TQ, an active molecule of Nigella sativa on two tumor cell lines, HCT116 and HT29. We began our study by testing the antioxidant and antiproliferative effects of TQ on the two adenocarcinoma cell lines HCT116 and HT29. Our results show that QT exhibits strong DDPH radical scavenging activity. This result is confirmed by the ABST test and shows an antioxidant effect that is very similar to vitamin C, reputed to be very antioxidant. The antiproliferative effect of TQ has been tested and confirmed by the MTT test. This antiproliferative effect has no effect on healthy cells, which proves that this substance can be consumed without danger, even at high doses, to our health. Many studies proved our results and showed the antiproliferative effect of this compound on colorectal cells [22].
Some studies have shown that certain spices with antioxidant properties also have a cytotoxic effect [23]. Other studies even prove a pro-apoptotic [22] and anti-proliferative effect of curcumin and TQ [24-25]. This study showed that the combination of TQ with other compounds seems to be more efficient on CRC [25]. The measurement of the amount of LDH in the culture medium provided us with excellent evidence of the cytotoxic effect of TQ on cancer cells. Hoechst staining of TQ-treated tumor cells showed ultra structural changes typical of these cells and the appearance of dense and/or fragmented nuclei, whereas no significant nuclear fragmentation was observed in the control group. Numerous studies have shown similar effect with different concentration [23]. The originality of our work is that we show, in the same study, the antioxidant and antiproliferative effect of TQ on cancerous cells while it remains without effect on normal cells even if it is used in high doses.
Conflicts of Interest
We want to draw the publisher’s attention that this work is an original idea of our laboratories ‘team and that it only responds to scientific interest. No member of the team was paid for this work. The research was supported by the laboratories which receives public funds from the Ministry of Higher Education of Tunisia.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Acknowledgment
We thank Mr. sami trabelsi for his help in the english version.
References
- Soumya T, Jayasree PR, Deepak M, Manish Kumar PR (2020) Chemical composition, antioxidant and antiproliferative activities of essential oil from rhizome and leaves of Curcuma mutabilis Škorničk. M Sabu & Prasanthk (Eds) endemic to Western Ghats of India. Nat Prod Res 34(16): 2336-2340.
- Yan M, Vemu B, Veenstra J, Petiwala SM, Jeremy J, et al. (2019) H HHS.
- Zheng J, Zhou Y, Li Y, Xu DP, Li S, et al. (2016) Spices for prevention and treatment of cancers. Nutrients 8(8): 495.
- Zhu D, Shao M, Yang J, Fang M, Liu S, et al. (2020) Curcumin Enhances Radiosensitization of Nasopharyngeal Carcinoma via Mediating Regulation of Tumor Stem-like Cells by a CircRNA Network. J Cancer 11(8): 2360-2370.
- Battino M, Forbes Hernandez T, Gasparrini M, Afrin S, Cianciosi D, et al. (2019) Relevance of functional foods in the Mediterranean diet: the role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit Rev Food Sci Nutr 59(6): 893: 920.
- Rajkumari S, Sanatombi K (2017) Nutritional value, phytochemical composition, and biological activities of edible Curcuma species: A Review. Int J Food Pro 20(sup3): S2668-S2687.
- Zaïri A, Nour S, Mhamdi N, Bennani M, Bergaoui I, et al. (2018) Antioxidant, Antimicrobial and the Phenolic Content of Infusion, Decoction and Methanolic Extracts of Thyme and Rosmarinus Curr Pharm Biotechnol 19(7): 590-599.
- Bianchi A (2015) The Mediterranean aromatic plants and their culinary use. Nat Prod Res 29(3): 201-206.
- Nur WWB Tajuddin M, Lajis NH, Abas F, Othman I, Rakesh N, et al. (2019) Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer 11(12): 1-29.
- Zuniga KE, Parma DL, Muñoz E, Spaniol M, Wargovich M, et al. (2019) Dietary intervention among breast cancer survivors increased adherence to a Mediterranean-style, anti-inflammatory dietary pattern: the Rx for Better Breast Health Randomized Controlled Trial. Breast Cancer Res Treat 173(1): 145-154.
- Reinholds I, Pougajeva I, Bavrins k, Kouckovska G, Bartkevics V, et al. (2017) Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit Contam Part B Surveill 10(1): 5-14.
- Kubatka P, Sona U, Martin K, Karol K, Marek S, et al. (2019) Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro. Int J Mol Sci 20(7): 1749.
- Al Menhali A, Al Rumaihi A, Al mohamed H, Al mazrooey H, Al shamlan M, et al. (2015) Thymus vulgaris (Thyme) Inhibits Proliferation, Adhesion, Migration and Invasion of Human Colorectal Cancer Cells. J med food 18(1): 54-59.
- Kartal N, Skmen M, Tepe B, Polissiou M, Sokmen A, et al. (2007) Investigation of the antioxidant properties of Ferula orientalis L. using a suitable extraction procedure. Food Chem 100(2): 584-589.
- Magalhaes LM, Marcela A, Segundo MA, Reis S, Lima JLFC, et al. (2008) Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta 613(1): 1-19.
- Yue GGL, Kwok HF, Lee JKM, Jiang L, Wong ECW, et al. (2016) Combined therapy using bevacizumab and turmeric ethanolic extract (with absorbable curcumin) exhibited beneficial efficacy in colon cancer mice. Pharmacol Res 111: 43-57.
- Farah B, Alissar M, Maamoun F, Hala El Ouweini, Miran A Jaffa, et al. (2020) Thymoquinone induces apoptosis and DNA damage in 5-Fluorouracil-resistant colorectal cancer stem/progenitor cells. Oncotarget 11(31): 2959-2972.
- Rana K, Md Hassan H, Rajaa F, Sandra R (2016) Thymoquinone from Nigella sativa Seeds Promotes the Antitumor Activity of Noncytotoxic Doses of Topotecan in Human Colorectal Cancer Cells in Vitro. Planta Med 82(4): 312-321.
- Mehra K, Berkowitz A, Sanft T (2017) Diet, Physical Activity, and Body Weight in Cancer Survivorship, Med Clin North Am 101(6): 1151-1165.
- Russo GI, Solinas T, Urzi D, Privitera S, Campisi D, et al. (2019) Adherence to Mediterranean diet and prostate cancer risk in Sicily: population-based case–control study. Int J Imp Res 31(4): 269-275.
- Alkhader E, J Roberts C, Rosli R, H Yuen K, Seow EK, et al. (2018) Pharmacokinetic and anti-colon cancer properties of curcumin-containing chitosan-pectinate composite nanoparticles. J Biomater Sci Polym Ed 29(18): 2281-2298.
- Lima FT, Viviane S, Gabriel S, Guilherme ST, Carlos RP, et al. (2018) The curcumin analog CH-5 Exerts Anticancer Effects In Human Osteosarcoma Cells via Modulation of Transcription Factors p53/Sp1. Int J Mol Sci 19(7): 1909.
- Fridlender M, Kapulnik Y, Koltai H (2015) Plant derived substances with anti-cancer activity: From folklore to practice Frontiers in Plant Science. Front Plant Sci 6: 799.
- Luthra PM, Lal N (2016) Prospective of curcumin, a pleiotropic signalling molecule from Curcuma longa in the treatment of Glioblastoma. Eur J Med Chem 109: 23-35.
- Bennani M, Zaïri A, Tabrelsi M (2021) Comparative Effects Between the Commercial and Homemade Curcuma on HCT116 Adenocarcinoma Cell Line. AJBSR 14(2): 213-218.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.